Extraction of Important Elements from their Ores
Extraction of Important Elements from their Ores: Overview
This Topic covers sub-topics such as Extraction of Gold, Extraction of Silver, Extraction of Chromium from Chromite Ore, Extraction of Manganese from Pyrolusite and, Extraction of Important Elements from their Ores
Important Questions on Extraction of Important Elements from their Ores
The carbon-based reduction method is NOT used for the extraction of
(a) tin from
(b) iron from
(c) aluminium from alumina
(d) magnesium from
In the cyanide extraction process of silver from argentite ore, the oxdising and reducing agents used are :
A metal obtained by a hydrometallurgical operation is:
The following flow diagram represents the extraction of magnesium from sea water.
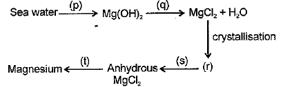
Which of the following option describes the correct reactants, products and reaction conditions?
In the equation,
Identify the metal ().
Silver is obtained from by reaction with:
Leaching the silver and gold metal with -:
In the extraction of silver from argentite air is passed through aqueous solution because:
Chemical reduction method is not used for:
and are added to fused in the electrolysis of , because:
Which of the following option represents the species and in the leaching of :
Which process is used to extract from commercial lead?
Leaching of silver is carried out by heating it with a dilute solution of
If dichloromethane (DCM) and water are used for differential extraction, then which of the following statements is correct?
Which of the following reactions is incorrect?
,Hence A and B are -
in the presence of produces a pink coloured solution when treated with The pink colour is due to the formation of
Which of the following reagent(s) can liberate at least one gaseous product with solid ?
Which of the following metal is extracted by the amalgamation process?
Sulphide mineral
In the above reaction, M can be -
